 |
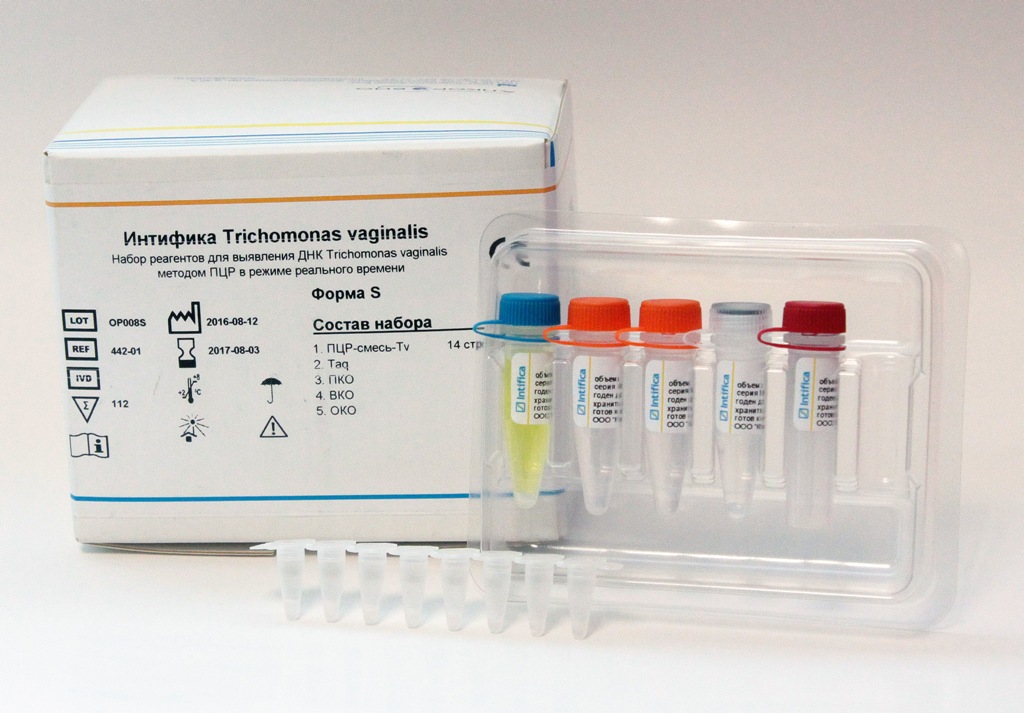 |
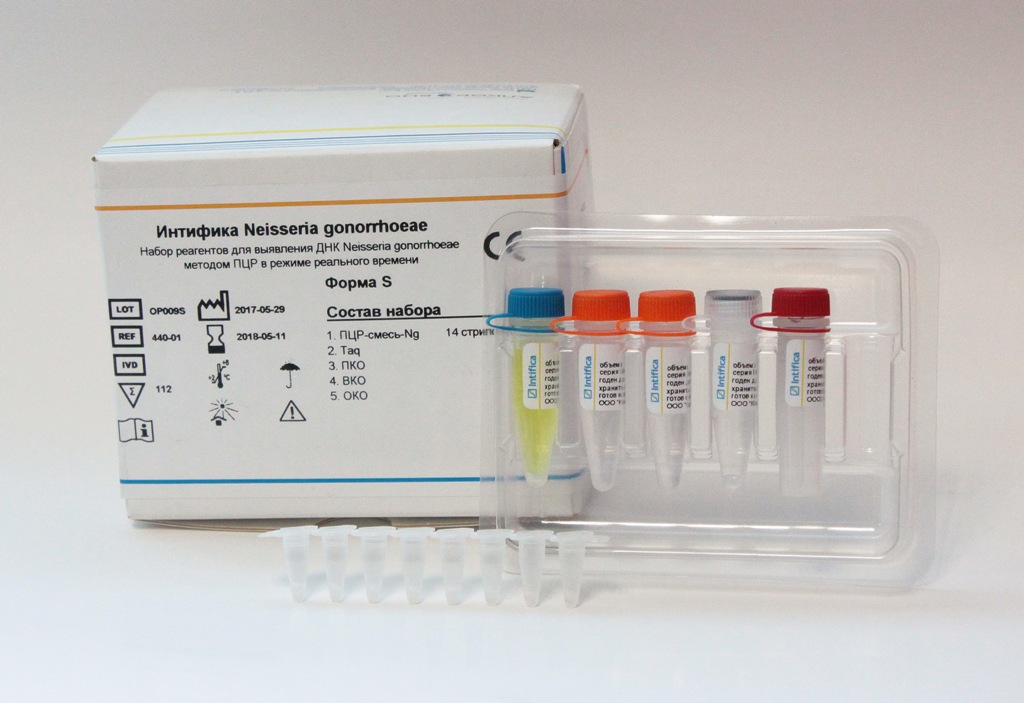
In the middle of December, the Alkor Bio Group of Companies, a developer and manufacturer of test-systems for laboratory diagnostics using ELISA and PCR, received registration certificates from the Russian Federal Health Service for Health Care Surveillance for their new kits: “Intifica Neisseria gonorrhoeae” for real-time PCR detection of gonorrhea and “Intifica Trichomonas vaginalis "to detect Trichomonas DNA by PCR in real time. The kits were developed in the PCR laboratory of Alkor Bio Group of Companies.
The kits for the qualitative determination of the DNA of these pathogens in nucleic acid preparations obtained using the DNA extraction kit produced by Alkor Bio from clinical specimens of smears / scrapings from the mucous membranes of the urogenital tract (urethra, cervical canal and vagina) and samples of the first urine using PCR in real time. PCR with the detection of amplification products in real time allows the diagnosis of infectious diseases and, in some cases, differential diagnosis and monitoring of the effectiveness of the treatment. The high sensitivity and relative simplicity of execution allows the PCR to be widely used for the diagnosis of STIs.
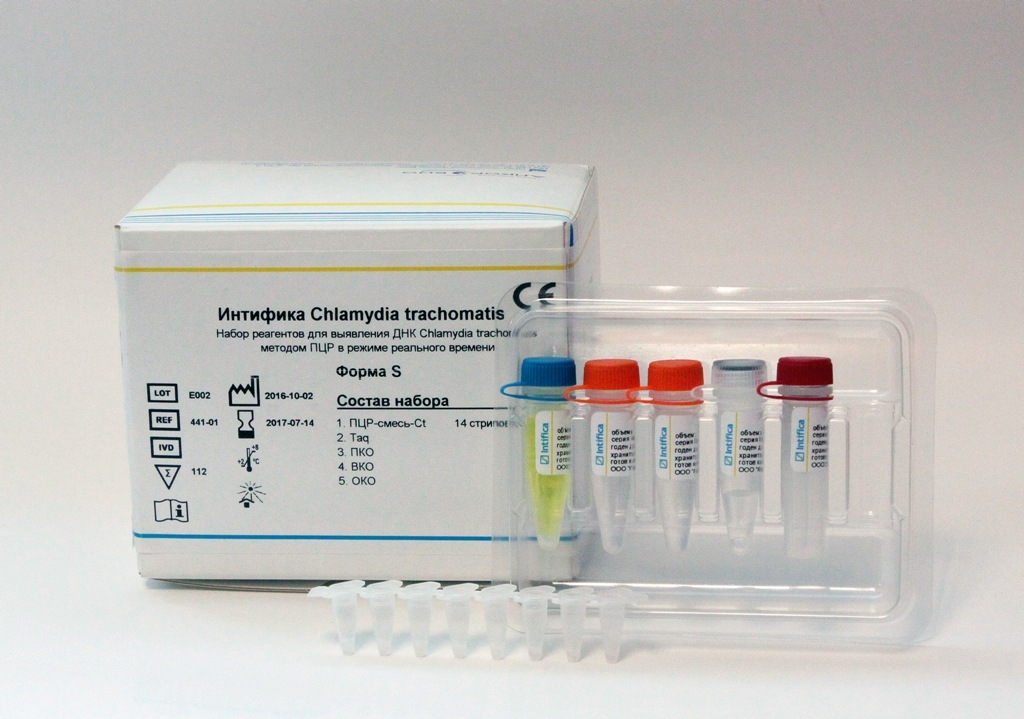 Recall that earlier the Alkor Bio Group of Companies received a registration certificate from the Roszdravnadzor of the Russian Federation for the “Intifica Chlamydia trachomatis” kit for detecting chlamydia DNA by PCR in real time.
Recall that earlier the Alkor Bio Group of Companies received a registration certificate from the Roszdravnadzor of the Russian Federation for the “Intifica Chlamydia trachomatis” kit for detecting chlamydia DNA by PCR in real time.
According to statistics, about 250 million people are infected every year in the world with sexually transmitted infections (STIs). Trichomoniasis, gonococcal and chlamydial infections are among the most common STIs. These infections are unconditional pathogens and can lead to serious negative consequences for human health. The key to successful treatment of gonorrhea, trichomoniasis and chlamydia is timely and accurate diagnosis.
Currently, Alkor Bio Group of Companies, produces kits in the direction of Infection Diagnostics: kits of reagents for detecting HIV, hepatitis B and C, infections of the TORCH complex, syphilis, trichomoniasis, gonococcal and chlamydial infections. In total, the company’s test system for ELISA and PCR contains more than a hundred items.