 |
 |
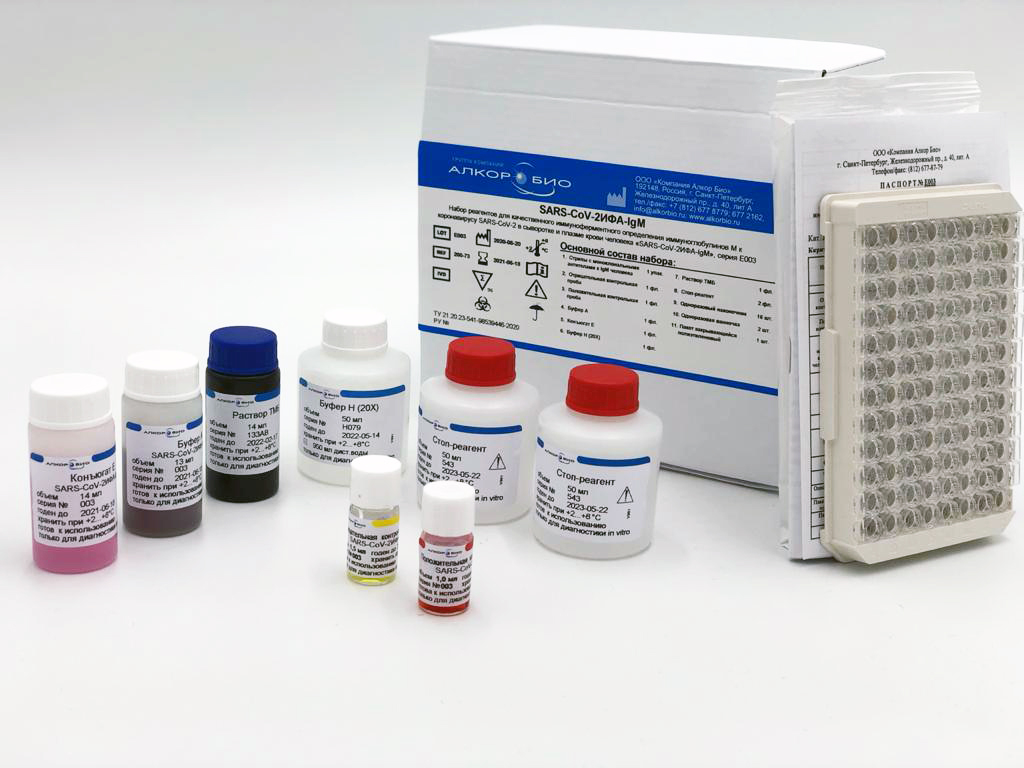 In the middle of September, the Alkor Bio Group of Companies (St. Petersburg), a Russian developer and manufacturer of test-systems and equipment for laboratory diagnostics by ELISA, IHLA and PCR methods, received a registration certificate from the Federal Service for Surveillance in Healthcare (Roszdravnadzor) for new kit "SARS-CoV-2 ELISA-IgM" for the qualitative enzyme immunoassay for the determination of immunoglobulins M to the SARS-CoV-2 in human serum and plasma.
In the middle of September, the Alkor Bio Group of Companies (St. Petersburg), a Russian developer and manufacturer of test-systems and equipment for laboratory diagnostics by ELISA, IHLA and PCR methods, received a registration certificate from the Federal Service for Surveillance in Healthcare (Roszdravnadzor) for new kit "SARS-CoV-2 ELISA-IgM" for the qualitative enzyme immunoassay for the determination of immunoglobulins M to the SARS-CoV-2 in human serum and plasma.
Two research laboratories of the Alkor Bio took part in the development of the new test system - the laboratory for the development of ELISA test systems and the biochemical laboratory.
Antibody determination allows confirmation of SARS-CoV-2 infection in patients with typical symptoms and, in suspicious cases, no symptoms. Specific class M antibodies appear 3-5 days after the onset of the disease. The functional purpose of the SARS-CoV-2 ELISA-IgM kit is the identification of class M immunoglobulins to the SARS-CoV-2 in human serum and plasma, detection of the early and acute phases of COVID-19.
The new kit is highly sensitive and specific, there is no preliminary dilution of samples is required. An indication occurs when the sample is added (the buffer color changes when serum is added to it); it is possible to automate all stages of the analysis.
Trim level of the kit of the SARS-CoV-2IFA-IgM allows setting in manual mode and on Alisei Q.S. The analyzer "Alisei" has a fully automated analysis process: adding reagents, incubation, washing, measuring optical density, processing the results. The program "SARS-CoV-2 ELISA-IgM" is entered into the analyzer's memory. It is possible to use other automatic analyzers for ELISA.
During tests of the SARS-CoV-2 ELISA-IgM kit, the absence of cross-reactions was shown in samples obtained from patients with confirmed hepatitis B, hepatitis C, from HIV-infected patients, from patients with metapneumovirus and respiratory syncytial virus, virus Epstein-Barr, from patients with influenza and parainfluenza, adenovirus infection, rhinovirus infection, enterovirus infection, with bacterial pneumonia (caused by Chlamydia pneumoniae, Mycoplasma pneumoniae, Streptococcus pneumoniae) and with tuberculosis.